Recommended dosing schedule and modifications
Manage ARs with dose delays, reductions, and discontinuations1*
-
Assess for peripheral neuropathy and obtain complete blood cell counts prior to each dose
-
Do not administer HALAVEN® on Day 1 or Day 8 in patients with ≥Grade 3 neutropenia,† ≥Grade 2 thrombocytopenia,‡ or Grade 3/4 nonhematologic toxicities
-
The Day 8 dose may be delayed for up to 1 week in patients with toxicities
-
If toxicities resolve or improve to Grade 2 or less by Day 15, administer at a reduced dose and initiate the next cycle no sooner than 2 weeks later
-
If toxicities do not resolve or improve to Grade 2 or less by Day 15, omit the dose
-
-
If a dose has been delayed for toxicities that have recovered to a severity of Grade 2 or less, resume at the recommended reduced dose
-
If a dose has been reduced due to toxicities, do not re-escalate
PATIENTS WHO RECEIVED DOSE DELAYS, DOSE REDUCTIONS, OR DISCONTINUED THERAPY WERE INCLUDED IN THE OVERALL SURVIVAL ANALYSIS OF EMBRACE2
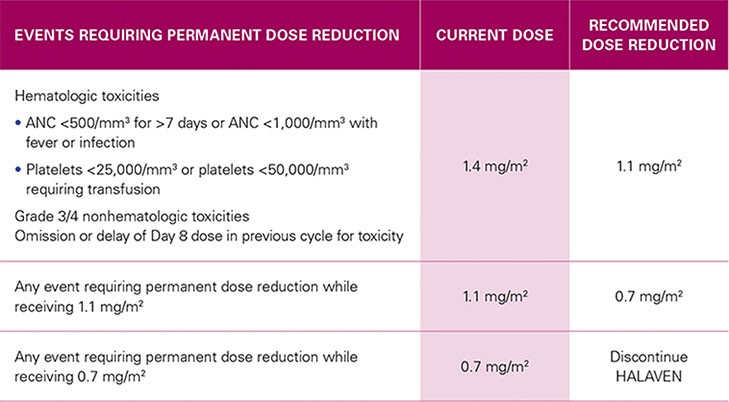
RECOMMENDED DOSE REDUCTIONS1*
| EVENTS REQUIRING PERMANENT DOSE REDUCTION | CURRENT DOSE |
RECOMMENDED DOSE REDUCTION |
|---|---|---|
Hematologic toxicities
Grade 3/4 nonhematologic toxicities |
1.4 mg/m2 | 1.1 mg/m2 |
| Any event requiring permanent dose reduction while receiving 1.1 mg/m2 | 1.1 mg/m2 | 0.7 mg/m2 |
| Any event requiring permanent dose reduction while receiving 0.7 mg/m2 | 0.7 mg/m2 | Discontinue HALAVEN |
EMBRACE=Eisai Metastatic Breast Cancer Study Assessing
Physician's Choice versus E7389 (Eribulin); ANC=absolute neutrophil count;
TPC=Treatment of Physician's Choice.
*Toxicities graded in accordance with National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE) version 3.0.1
†Greater than or equal to Grade 3 neutropenia=ANC <1,000/mm3.3
‡Greater than or equal to Grade 2 thrombocytopenia=platelets <75,000/mm3.3
Patients in the HALAVEN arm received a median of 5 cycles of therapy (range 1-23) in the pivotal Phase III trial1
-
The median duration of exposure was 118 days in the HALAVEN arm and 63 days in the TPC arm
Review the Recommended Dosing Schedule and Modifications schematic
Best viewed on flat surface in landscape view.
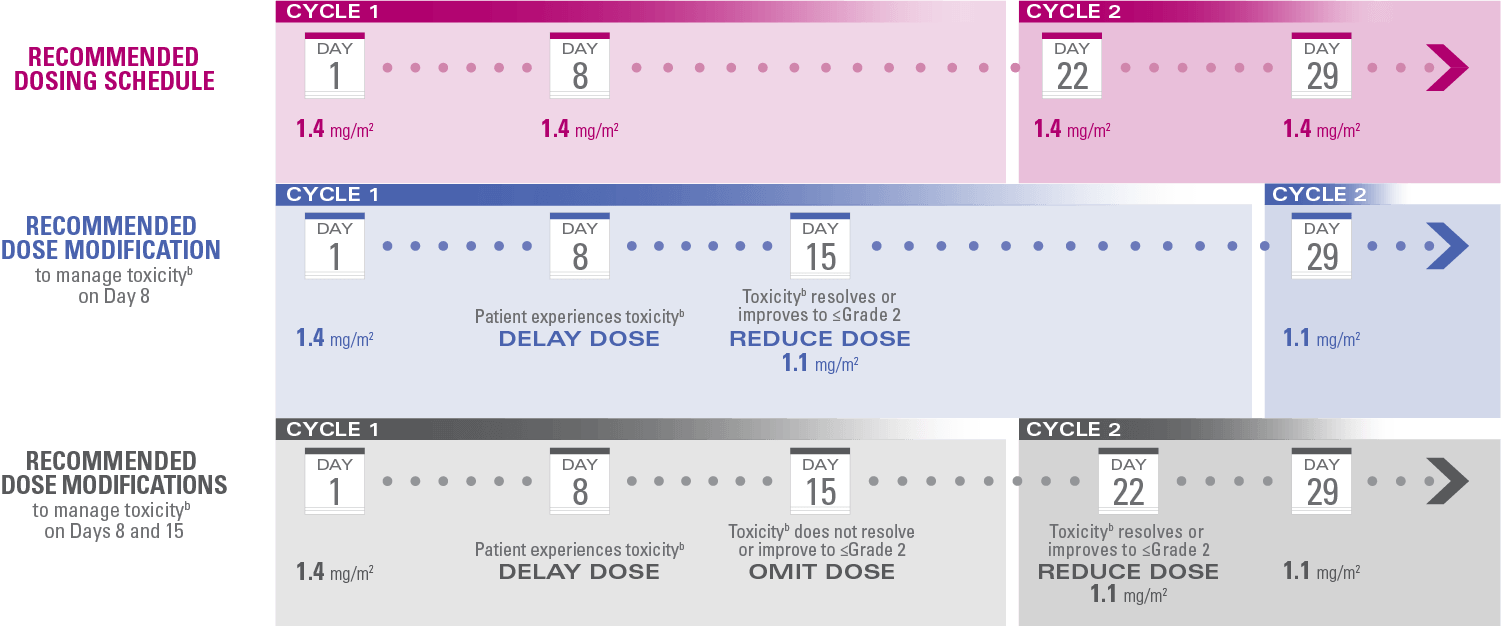
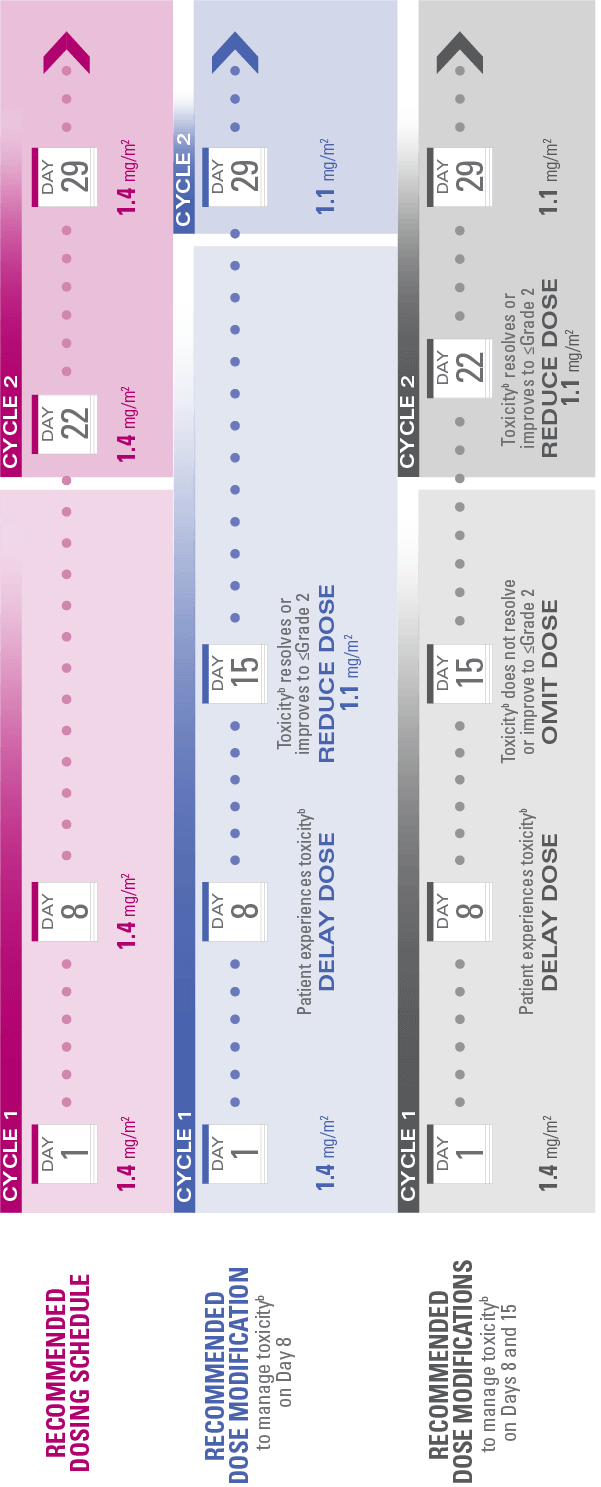
aToxicities graded in accordance with National Cancer Institute Common Terminology Criteria for Adverse Events version 3.0.3
bToxicities include ≥Grade 3 neutropenia (ANC <1,000/mm3), ≥Grade 2 thrombocytopenia (platelets <75,000/mm3), or Grade 3/4 nonhematologic toxicities.1,3
-
For any event requiring permanent dose reduction while receiving 1.1 mg/m2, reduce to 0.7 mg/m2 of HALAVEN1
-
For any event requiring permanent dose reduction while receiving 0.7 mg/m2, discontinue HALAVEN1
Indications
Metastatic Breast Cancer
HALAVEN (eribulin mesylate) Injection is indicated for the treatment of patients with metastatic breast cancer (mBC) who have previously received at least 2 chemotherapeutic regimens for the treatment of metastatic disease. Prior therapy should have included an anthracycline and a taxane in either the adjuvant or metastatic setting.
Liposarcoma
HALAVEN is indicated for the treatment of patients with unresectable or metastatic liposarcoma who have received a prior anthracycline-containing regimen.
Important Safety Information
Warnings and Precautions
Neutropenia: Severe neutropenia (ANC <500/mm3) lasting >1 week occurred in 12% of patients with mBC and liposarcoma or leiomyosarcoma. Febrile neutropenia occurred in 5% of patients with mBC and 2 patients (0.4%) died from complications. Febrile neutropenia occurred in 0.9% of patients with liposarcoma or leiomyosarcoma, and fatal neutropenic sepsis occurred in 0.9% of patients. Patients with mBC with elevated liver enzymes >3 × ULN and bilirubin >1.5 × ULN experienced a higher incidence of Grade 4 neutropenia and febrile neutropenia than patients with normal levels. Monitor complete blood cell counts prior to each dose, and increase the frequency of monitoring in patients who develop Grade 3 or 4 cytopenias. Delay administration and reduce subsequent doses in patients who experience febrile neutropenia or Grade 4 neutropenia lasting >7 days.
Peripheral Neuropathy: Grade 3 peripheral neuropathy occurred in 8% of patients with mBC (Grade 4=0.4%) and 22% developed a new or worsening neuropathy that had not recovered within a median follow-up duration of 269 days (range 25-662 days). Neuropathy lasting >1 year occurred in 5% of patients with mBC. Grade 3 peripheral neuropathy occurred in 3.1% of patients with liposarcoma and leiomyosarcoma receiving HALAVEN and neuropathy lasting more than 60 days occurred in 58% (38/65) of patients who had neuropathy at the last treatment visit. Patients should be monitored for signs of peripheral motor and sensory neuropathy. Withhold HALAVEN in patients who experience Grade 3 or 4 peripheral neuropathy until resolution to Grade 2 or less.
Embryo-Fetal Toxicity: HALAVEN can cause fetal harm when administered to a pregnant woman. Advise females of reproductive potential to use effective contraception during treatment with HALAVEN and for at least 2 weeks following the final dose. Advise males with female partners of reproductive potential to use effective contraception during treatment with HALAVEN and for 3.5 months following the final dose.
QT Prolongation: Monitor for prolonged QT intervals in patients with congestive heart failure, bradyarrhythmias, drugs known to prolong the QT interval, and electrolyte abnormalities. Correct hypokalemia or hypomagnesemia prior to initiating HALAVEN and monitor these electrolytes periodically during therapy. Avoid in patients with congenital long QT syndrome.
Adverse Reactions
In patients with mBC receiving HALAVEN, the most common adverse reactions (≥25%) were neutropenia (82%), anemia (58%), asthenia/fatigue (54%), alopecia (45%), peripheral neuropathy (35%), nausea (35%), and constipation (25%). Febrile neutropenia (4%) and neutropenia (2%) were the most common serious adverse reactions. The most common adverse reaction resulting in discontinuation was peripheral neuropathy (5%).
In patients with liposarcoma and leiomyosarcoma receiving HALAVEN, the most common adverse reactions (≥25%) reported in patients receiving HALAVEN were fatigue (62%), nausea (41%), alopecia (35%), constipation (32%), peripheral neuropathy (29%), abdominal pain (29%), and pyrexia (28%). The most common (≥5%) Grade 3-4 laboratory abnormalities reported in patients receiving HALAVEN were neutropenia (32%), hypokalemia (5.4%), and hypocalcemia (5%). Neutropenia (4.9%) and pyrexia (4.5%) were the most common serious adverse reactions. The most common adverse reactions resulting in discontinuation were fatigue and thrombocytopenia (0.9% each).
Use in Specific Populations
Lactation: Because of the potential for serious adverse reactions in breastfed infants from eribulin mesylate, advise women not to breastfeed during treatment with HALAVEN and for 2 weeks after the final dose.
Hepatic and Renal Impairment: A reduction in starting dose is recommended for patients with mild or moderate hepatic impairment and/or moderate or severe renal impairment.
Indications
Metastatic Breast Cancer
HALAVEN (eribulin mesylate) Injection is indicated for the treatment of patients with metastatic breast cancer (mBC) who have previously received at least 2 chemotherapeutic regimens for the treatment of metastatic disease. Prior therapy should have included an anthracycline and a taxane in either the adjuvant or metastatic setting.
Liposarcoma
HALAVEN is indicated for the treatment of patients with unresectable or metastatic liposarcoma who have received a prior anthracycline-containing regimen.
Indications
Metastatic Breast Cancer
HALAVEN (eribulin mesylate) Injection is indicated for the treatment of patients with metastatic breast cancer (mBC) who have previously received at least 2 chemotherapeutic regimens for the treatment of metastatic disease. Prior therapy should have included an anthracycline and a taxane in either the adjuvant or metastatic setting.
Liposarcoma
HALAVEN is indicated for the treatment of patients with unresectable or metastatic liposarcoma who have received a prior anthracycline-containing regimen.
Important Safety Information
Warnings and Precautions
Neutropenia: Severe neutropenia (ANC <500/mm3) lasting >1 week occurred in 12% of patients with mBC and liposarcoma or leiomyosarcoma. Febrile neutropenia occurred in 5% of patients with mBC and 2 patients (0.4%) died from complications. Febrile neutropenia occurred in 0.9% of patients with liposarcoma or leiomyosarcoma, and fatal neutropenic sepsis occurred in 0.9% of patients. Patients with mBC with elevated liver enzymes >3 × ULN and bilirubin >1.5 × ULN experienced a higher incidence of Grade 4 neutropenia and febrile neutropenia than patients with normal levels. Monitor complete blood cell counts prior to each dose, and increase the frequency of monitoring in patients who develop Grade 3 or 4 cytopenias. Delay administration and reduce subsequent doses in patients who experience febrile neutropenia or Grade 4 neutropenia lasting >7 days.
Peripheral Neuropathy: Grade 3 peripheral neuropathy occurred in 8% of patients with mBC (Grade 4=0.4%) and 22% developed a new or worsening neuropathy that had not recovered within a median follow-up duration of 269 days (range 25-662 days). Neuropathy lasting >1 year occurred in 5% of patients with mBC. Grade 3 peripheral neuropathy occurred in 3.1% of patients with liposarcoma and leiomyosarcoma receiving HALAVEN and neuropathy lasting more than 60 days occurred in 58% (38/65) of patients who had neuropathy at the last treatment visit. Patients should be monitored for signs of peripheral motor and sensory neuropathy. Withhold HALAVEN in patients who experience Grade 3 or 4 peripheral neuropathy until resolution to Grade 2 or less.
Embryo-Fetal Toxicity: HALAVEN can cause fetal harm when administered to a pregnant woman. Advise females of reproductive potential to use effective contraception during treatment with HALAVEN and for at least 2 weeks following the final dose. Advise males with female partners of reproductive potential to use effective contraception during treatment with HALAVEN and for 3.5 months following the final dose.
QT Prolongation: Monitor for prolonged QT intervals in patients with congestive heart failure, bradyarrhythmias, drugs known to prolong the QT interval, and electrolyte abnormalities. Correct hypokalemia or hypomagnesemia prior to initiating HALAVEN and monitor these electrolytes periodically during therapy. Avoid in patients with congenital long QT syndrome.
Adverse Reactions
In patients with mBC receiving HALAVEN, the most common adverse reactions (≥25%) were neutropenia (82%), anemia (58%), asthenia/fatigue (54%), alopecia (45%), peripheral neuropathy (35%), nausea (35%), and constipation (25%). Febrile neutropenia (4%) and neutropenia (2%) were the most common serious adverse reactions. The most common adverse reaction resulting in discontinuation was peripheral neuropathy (5%).
In patients with liposarcoma and leiomyosarcoma receiving HALAVEN, the most common adverse reactions (≥25%) reported in patients receiving HALAVEN were fatigue (62%), nausea (41%), alopecia (35%), constipation (32%), peripheral neuropathy (29%), abdominal pain (29%), and pyrexia (28%). The most common (≥5%) Grade 3-4 laboratory abnormalities reported in patients receiving HALAVEN were neutropenia (32%), hypokalemia (5.4%), and hypocalcemia (5%). Neutropenia (4.9%) and pyrexia (4.5%) were the most common serious adverse reactions. The most common adverse reactions resulting in discontinuation were fatigue and thrombocytopenia (0.9% each).
Use in Specific Populations
Lactation: Because of the potential for serious adverse reactions in breastfed infants from eribulin mesylate, advise women not to breastfeed during treatment with HALAVEN and for 2 weeks after the final dose.
Hepatic and Renal Impairment: A reduction in starting dose is recommended for patients with mild or moderate hepatic impairment and/or moderate or severe renal impairment.
References: 1. HALAVEN [package insert]. Nutley, NJ: Eisai Inc. 2. Data on file, Eisai Inc. 3. National Cancer Institute. Cancer Therapy Evaluation Program, Common Terminology Criteria for Adverse Events v3.0. NIH Publication # 03-5410. http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcaev3.pdf. Published March 31, 2003. Updated August 9, 2006. Accessed January 31, 2018.

