Retrospective study of HALAVEN® in metastatic breast cancer patients in oncology centers in the United States1
Objective
To describe the real-world treatment patterns with HALAVEN in patients with ≥3L mBC, including a subgroup with the TNBC subtype, when treated in the United States in accordance with the USPI for HALAVEN.*
Design1
This was a retrospective multi-site patient chart review study of adult females who initiated HALAVEN for mBC between January 1, 2011 and December 31, 2017. Data were extracted from patient charts by providers in the Cardinal Health Oncology Provider Extended Network.
This study required that only eligible HCPs within the Cardinal Health Network with eligible patients who were willing to participate, could provide data. In total, 46 oncologists provided data for 513 mBC patients.
Clinical outcomes
Clinical outcomes assessed included overall survival (OS) and progression-free survival (PFS). Safety data were not collected in this study.
No direct comparisons between results from the pivotal clinical trial and real-world data study should be made, as there could be potential differences in patient populations, patient characteristics, line of treatment use, follow-up duration, response assessment timing, frequency, and criteria that are used in clinical trials vs real-world settings. Because this is a single cohort study, no data on comparative therapies were included.
mBC=metastatic breast cancer; TNBC=triple negative breast cancer; USPI=United States prescribing information
*HALAVEN (eribulin mesylate) Injection is indicated for the treatment of patients with metastatic breast cancer (mBC) who have previously received at least 2 chemotherapeutic regimens for the treatment of metastatic disease. Prior therapy should have included an anthracycline and a taxane in either the adjuvant or metastatic setting.
Real-world evidence: Patient demographics and baseline characteristics1,2
Best viewed on flat surface in landscape view.
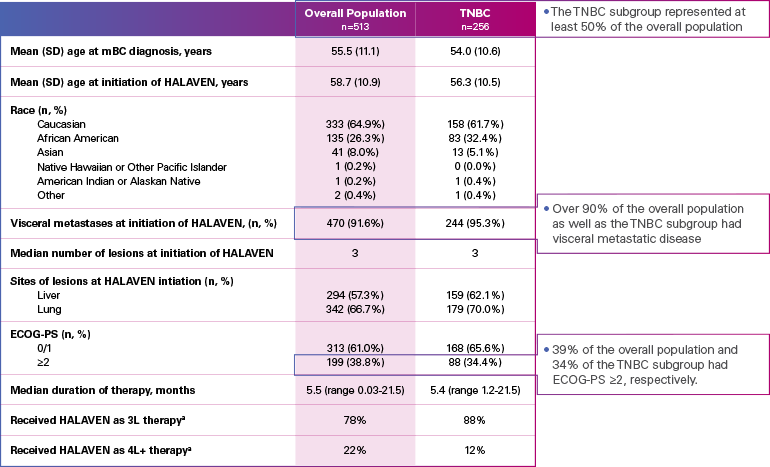
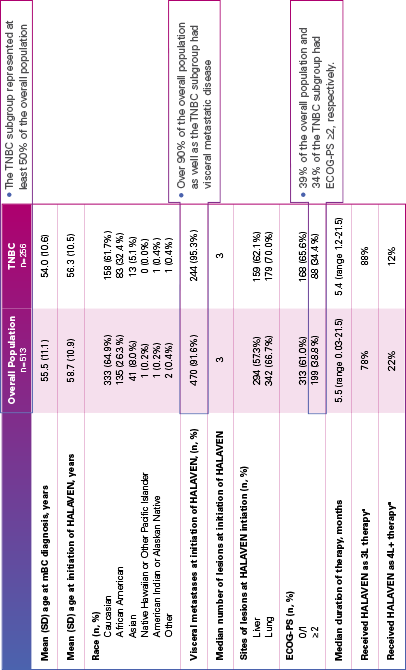
ECOG-PS=Eastern Cooperative Oncology Group performance status; mBC=metastatic breast cancer; SD=standard deviation; TNBC=triple negative breast cancer.
aInitiated HALAVEN between January 1, 2011 and December 31, 2017 after 2 prior chemotherapy regimens in the metastatic setting and prior to treatment in either the adjuvant or metastatic setting with an anthracycline and a taxane.
Limitation: The results of this real-world study should be interpreted with caution because of the potential for selection bias, since the study patient cohort represents only practices of physicians who agreed to participate in the study, and potential loss to follow-up during study period. No conclusions can be drawn.
Real-world evidence: Overall survival1
In this retrospective study:
- In the overall patient population (n=513), median OS was 10.6 months (95% Cl: 9.9-11.7)
- In the TNBC subgroup (n=256), median OS was 9.8 months (95% CI: 8.6-11.0)
ESTIMATED OS RATE IN OVERALL PATIENT POPULATION AND TNBC SUBGROUP
Best viewed on flat surface in landscape view.
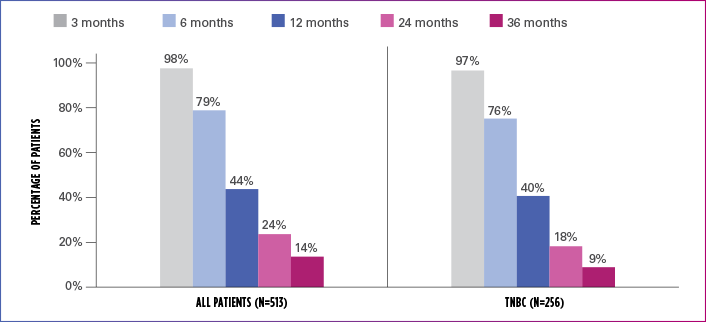
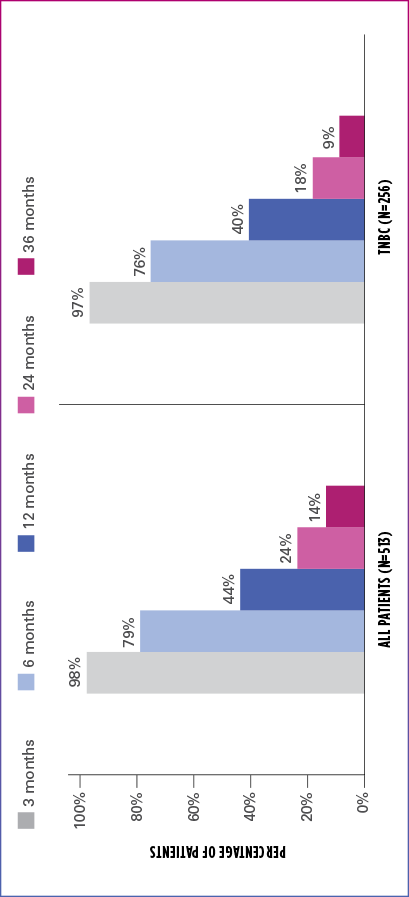
OS=overall survival; TNBC=triple negative breast cancer.
Limitation: The results of this real-world study should be interpreted with caution because of the potential for selection bias, since the study patient cohort represents only practices of physicians who agreed to participate in the study, and potential loss to follow-up during study period. No conclusions can be drawn.
Real-world evidence: Progression-free survival1
In this retrospective study:
- In the overall patient population (n=513), median PFS was 6.1 months (95% CI: 5.8-6.6)
- In the TNBC subgroup (n=256), median PFS was 5.8 months (95% CI: 5.1-6.4)
ESTIMATED PFS RATE IN OVERALL PATIENT POPULATION AND TNBC SUBGROUP
Best viewed on flat surface in landscape view.
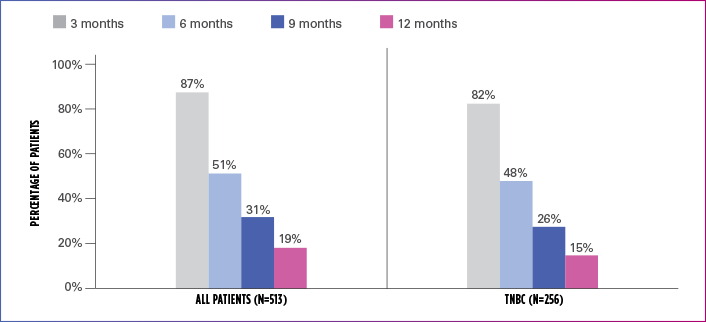
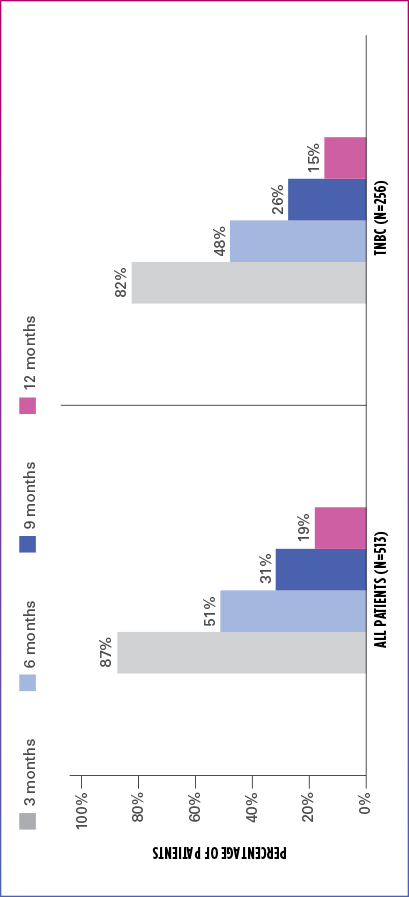
PFS=progression-free survival; TNBC=triple negative breast cancer.
Limitation: The results of this real-world study should be interpreted with caution because of the potential for selection bias, since the study patient cohort represents only practices of physicians who agreed to participate in the study, and potential loss to follow-up during study period. No conclusions can be drawn.
HALAVEN CLINICAL TRIAL RESULTS (EMBRACE)3
- Progression-Free Survival: PFS was a secondary endpoint in EMBRACE. Median PFS was 3.7 months with HALAVEN vs 2.2 months with TPC (HR=0.87 [95% CI: 0.71, 1.05])
Limitation: PFS did not show a statistically significant difference between HALAVEN and the TPC arm.
Indications
Metastatic Breast Cancer
HALAVEN (eribulin mesylate) Injection is indicated for the treatment of patients with metastatic breast cancer (mBC) who have previously received at least 2 chemotherapeutic regimens for the treatment of metastatic disease. Prior therapy should have included an anthracycline and a taxane in either the adjuvant or metastatic setting.
Liposarcoma
HALAVEN is indicated for the treatment of patients with unresectable or metastatic liposarcoma who have received a prior anthracycline-containing regimen.
Important Safety Information
Warnings and Precautions
Neutropenia: Severe neutropenia (ANC <500/mm3) lasting >1 week occurred in 12% of patients with mBC and liposarcoma or leiomyosarcoma. Febrile neutropenia occurred in 5% of patients with mBC and 2 patients (0.4%) died from complications. Febrile neutropenia occurred in 0.9% of patients with liposarcoma or leiomyosarcoma, and fatal neutropenic sepsis occurred in 0.9% of patients. Patients with mBC with elevated liver enzymes >3 × ULN and bilirubin >1.5 × ULN experienced a higher incidence of Grade 4 neutropenia and febrile neutropenia than patients with normal levels. Monitor complete blood cell counts prior to each dose, and increase the frequency of monitoring in patients who develop Grade 3 or 4 cytopenias. Delay administration and reduce subsequent doses in patients who experience febrile neutropenia or Grade 4 neutropenia lasting >7 days.
Peripheral Neuropathy: Grade 3 peripheral neuropathy occurred in 8% of patients with mBC (Grade 4=0.4%) and 22% developed a new or worsening neuropathy that had not recovered within a median follow-up duration of 269 days (range 25-662 days). Neuropathy lasting >1 year occurred in 5% of patients with mBC. Grade 3 peripheral neuropathy occurred in 3.1% of patients with liposarcoma and leiomyosarcoma receiving HALAVEN and neuropathy lasting more than 60 days occurred in 58% (38/65) of patients who had neuropathy at the last treatment visit. Patients should be monitored for signs of peripheral motor and sensory neuropathy. Withhold HALAVEN in patients who experience Grade 3 or 4 peripheral neuropathy until resolution to Grade 2 or less.
Embryo-Fetal Toxicity: HALAVEN can cause fetal harm when administered to a pregnant woman. Advise females of reproductive potential to use effective contraception during treatment with HALAVEN and for at least 2 weeks following the final dose. Advise males with female partners of reproductive potential to use effective contraception during treatment with HALAVEN and for 3.5 months following the final dose.
QT Prolongation: Monitor for prolonged QT intervals in patients with congestive heart failure, bradyarrhythmias, drugs known to prolong the QT interval, and electrolyte abnormalities. Correct hypokalemia or hypomagnesemia prior to initiating HALAVEN and monitor these electrolytes periodically during therapy. Avoid in patients with congenital long QT syndrome.
Adverse Reactions
In patients with mBC receiving HALAVEN, the most common adverse reactions (≥25%) were neutropenia (82%), anemia (58%), asthenia/fatigue (54%), alopecia (45%), peripheral neuropathy (35%), nausea (35%), and constipation (25%). Febrile neutropenia (4%) and neutropenia (2%) were the most common serious adverse reactions. The most common adverse reaction resulting in discontinuation was peripheral neuropathy (5%).
In patients with liposarcoma and leiomyosarcoma receiving HALAVEN, the most common adverse reactions (≥25%) reported in patients receiving HALAVEN were fatigue (62%), nausea (41%), alopecia (35%), constipation (32%), peripheral neuropathy (29%), abdominal pain (29%), and pyrexia (28%). The most common (≥5%) Grade 3-4 laboratory abnormalities reported in patients receiving HALAVEN were neutropenia (32%), hypokalemia (5.4%), and hypocalcemia (5%). Neutropenia (4.9%) and pyrexia (4.5%) were the most common serious adverse reactions. The most common adverse reactions resulting in discontinuation were fatigue and thrombocytopenia (0.9% each).
Use in Specific Populations
Lactation: Because of the potential for serious adverse reactions in breastfed infants from eribulin mesylate, advise women not to breastfeed during treatment with HALAVEN and for 2 weeks after the final dose.
Hepatic and Renal Impairment: A reduction in starting dose is recommended for patients with mild or moderate hepatic impairment and/or moderate or severe renal impairment.
Indications
Metastatic Breast Cancer
HALAVEN (eribulin mesylate) Injection is indicated for the treatment of patients with metastatic breast cancer (mBC) who have previously received at least 2 chemotherapeutic regimens for the treatment of metastatic disease. Prior therapy should have included an anthracycline and a taxane in either the adjuvant or metastatic setting.
Liposarcoma
HALAVEN is indicated for the treatment of patients with unresectable or metastatic liposarcoma who have received a prior anthracycline-containing regimen.
Indications
Metastatic Breast Cancer
HALAVEN (eribulin mesylate) Injection is indicated for the treatment of patients with metastatic breast cancer (mBC) who have previously received at least 2 chemotherapeutic regimens for the treatment of metastatic disease. Prior therapy should have included an anthracycline and a taxane in either the adjuvant or metastatic setting.
Liposarcoma
HALAVEN is indicated for the treatment of patients with unresectable or metastatic liposarcoma who have received a prior anthracycline-containing regimen.
Important Safety Information
Warnings and Precautions
Neutropenia: Severe neutropenia (ANC <500/mm3) lasting >1 week occurred in 12% of patients with mBC and liposarcoma or leiomyosarcoma. Febrile neutropenia occurred in 5% of patients with mBC and 2 patients (0.4%) died from complications. Febrile neutropenia occurred in 0.9% of patients with liposarcoma or leiomyosarcoma, and fatal neutropenic sepsis occurred in 0.9% of patients. Patients with mBC with elevated liver enzymes >3 × ULN and bilirubin >1.5 × ULN experienced a higher incidence of Grade 4 neutropenia and febrile neutropenia than patients with normal levels. Monitor complete blood cell counts prior to each dose, and increase the frequency of monitoring in patients who develop Grade 3 or 4 cytopenias. Delay administration and reduce subsequent doses in patients who experience febrile neutropenia or Grade 4 neutropenia lasting >7 days.
Peripheral Neuropathy: Grade 3 peripheral neuropathy occurred in 8% of patients with mBC (Grade 4=0.4%) and 22% developed a new or worsening neuropathy that had not recovered within a median follow-up duration of 269 days (range 25-662 days). Neuropathy lasting >1 year occurred in 5% of patients with mBC. Grade 3 peripheral neuropathy occurred in 3.1% of patients with liposarcoma and leiomyosarcoma receiving HALAVEN and neuropathy lasting more than 60 days occurred in 58% (38/65) of patients who had neuropathy at the last treatment visit. Patients should be monitored for signs of peripheral motor and sensory neuropathy. Withhold HALAVEN in patients who experience Grade 3 or 4 peripheral neuropathy until resolution to Grade 2 or less.
Embryo-Fetal Toxicity: HALAVEN can cause fetal harm when administered to a pregnant woman. Advise females of reproductive potential to use effective contraception during treatment with HALAVEN and for at least 2 weeks following the final dose. Advise males with female partners of reproductive potential to use effective contraception during treatment with HALAVEN and for 3.5 months following the final dose.
QT Prolongation: Monitor for prolonged QT intervals in patients with congestive heart failure, bradyarrhythmias, drugs known to prolong the QT interval, and electrolyte abnormalities. Correct hypokalemia or hypomagnesemia prior to initiating HALAVEN and monitor these electrolytes periodically during therapy. Avoid in patients with congenital long QT syndrome.
Adverse Reactions
In patients with mBC receiving HALAVEN, the most common adverse reactions (≥25%) were neutropenia (82%), anemia (58%), asthenia/fatigue (54%), alopecia (45%), peripheral neuropathy (35%), nausea (35%), and constipation (25%). Febrile neutropenia (4%) and neutropenia (2%) were the most common serious adverse reactions. The most common adverse reaction resulting in discontinuation was peripheral neuropathy (5%).
In patients with liposarcoma and leiomyosarcoma receiving HALAVEN, the most common adverse reactions (≥25%) reported in patients receiving HALAVEN were fatigue (62%), nausea (41%), alopecia (35%), constipation (32%), peripheral neuropathy (29%), abdominal pain (29%), and pyrexia (28%). The most common (≥5%) Grade 3-4 laboratory abnormalities reported in patients receiving HALAVEN were neutropenia (32%), hypokalemia (5.4%), and hypocalcemia (5%). Neutropenia (4.9%) and pyrexia (4.5%) were the most common serious adverse reactions. The most common adverse reactions resulting in discontinuation were fatigue and thrombocytopenia (0.9% each).
Use in Specific Populations
Lactation: Because of the potential for serious adverse reactions in breastfed infants from eribulin mesylate, advise women not to breastfeed during treatment with HALAVEN and for 2 weeks after the final dose.
Hepatic and Renal Impairment: A reduction in starting dose is recommended for patients with mild or moderate hepatic impairment and/or moderate or severe renal impairment.
References: 1. Mougalian SS, Kish JK, Zhang J, Liassou D, Feinberg BA. Effectiveness of eribulin in metastatic breast cancer: 10 years of real–world clinical experience in the United States. Adv Ther. 2021;38(5):2213-2225. 2. Data on file, Eisai Inc. 3. Cortes J, O'Shaughnessy J, Loesch D, et al; EMBRACE (Eisai Metastatic Breast Cancer Study Assessing Physician's Choice Versus E7389) investigators. Eribulin monotherapy versus treatment of physician's choice in patients with metastatic breast cancer (EMBRACE): a phase 3 open-label randomised study. Lancet. 2011;377(9769):914-923.

